Image Credit: Photo courtesy of Visby Medical.
Recent advancements in sexual health are making significant strides in accessible testing and treatment options for sexually transmitted diseases (STDs) in the United States. The U.S. Food and Drug Administration (FDA) has approved several at-home STD testing kits and new treatment medications, marking a pivotal moment for public health.
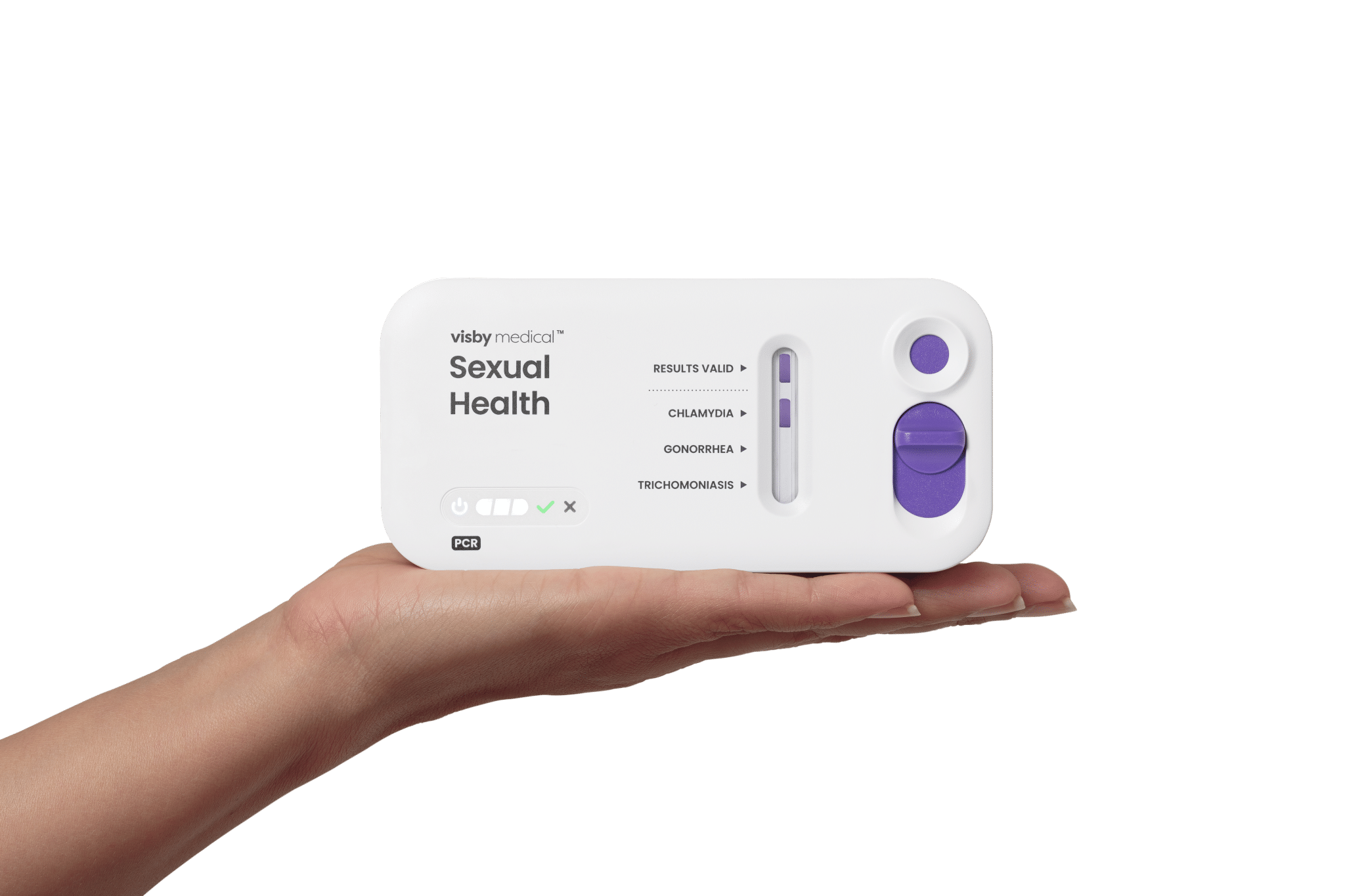
The FDA recently granted approval for Visby Medical’s at-home testing kit, which screens for gonorrhea, chlamydia, and trichomoniasis in women. This all-in-one test involves a simple vaginal swab and a diagnostic device that allows users to upload results to an app. Following this, individuals can access a telehealth consultation and receive prescriptions within hours. The accuracy of this test is reported to be around 98%, comparable to traditional lab results, and is priced at $150, although it is not currently covered by insurance.
In addition to advancements in testing, the FDA has also approved two new oral medications for gonorrhea treatment, named Nuzolvenc and Bluejepa. These new treatments offer alternatives to the previously standard injectable ceftriaxone, addressing concerns over drug-resistant strains of gonorrhea. The introduction of these medications is particularly significant, as it provides additional options in a landscape that has seen dwindling effective treatments.
Provisional data from the Centers for Disease Control and Prevention (CDC) indicates a downward trend in STD rates, including a decline in gonorrhea cases for three consecutive years. Contributing factors to this decrease may include reduced sexual activity among younger populations and increased access to at-home screening. However, experts warn that the shift toward home testing may complicate national infection tracking, as many public health databases depend on centralized lab reporting.
Challenges remain, particularly in terms of access and cost. While at-home testing provides convenience, the $150 price tag may pose a barrier for lower-income individuals. Additionally, funding cuts to public health agencies raise concerns about equitable access to these innovative solutions.
Overall, the recent FDA approvals represent a significant evolution in sexual health, providing individuals with more options for testing and treatment while highlighting ongoing challenges in public health access.
Check out the original article here: Source link